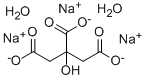
Product name:Trisodium citrate dihydrate
CAS:6132-04-3
Molecular Formula:C6H9Na3O9
Formula Weight:294.1
Specification:500g
Description:
Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as sodium citrate, though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor. For this reason, citrates of certain alkaline and alkaline earth metals (e.g. sodium and calcium citrates) are commonly known as sour salt (occasionally citric acid is erroneously termed sour salt).
Trisodium citrate dients are white, tasteless, briny and stable in air. Relative density 1.857(23.5℃). The water of crystallization is lost at 150℃ and decomposed by further heating. Soluble in water, aqueous solution pH is about 8, difficult to dissolve in ethanol. Made from sodium hydroxide or sodium carbonate neutralized, concentrated and crystallized in citric acid.
It is used as anticoagulants, expectorants and diuretics in the pharmaceutical industry. It is also used in the preparation, injection and pharmaceutical products.
Trisodium citrate dients are the most important citrate salts. It is mainly produced from starch through fermentation to citric acid and neutralized with alkali. It has the following excellent properties:
(1) safe and non-toxic performance. Because the raw material of sodium citrate is basically from food, it is absolutely safe and reliable, and will not harm human health. The food and agriculture organization of the United Nations and the world health organization do not limit their daily intake, it can be considered as drug-free.
(2) biodegradability. After the sodium citrate is diluted by a large amount of water in nature, part of it becomes citric acid and the two coexist in the same system. Citric acid is easily biodegraded by oxygen, heat, light, bacteria and microorganisms in water. The decomposition pathway is generally converted into carbon dioxide and water by aconitonic acid, itaconic acid and linconic anhydride.
(3) metal ion complexing ability. Sodium citrate has good complexing ability for metal ions such as Ca2+ and Mg2+, and for other metal ions such as Fe2+ plasma.
(4) excellent solubility, and the solubility increases with the increase of water temperature.
(5) good pH adjustment and buffering performance. Sodium citrate is a weak acid and strong base salt, which can be combined with citric acid to form a strong pH buffer. In addition, sodium citrate has excellent retarding and stabilizing properties.

